When one psychiatric medication isn’t enough, doctors often add another. This isn’t experimental-it’s standard practice for people with treatment-resistant depression, bipolar disorder, or severe anxiety. But when you switch from a brand-name drug to a generic version, especially in a combination, things can go wrong-sometimes quietly, sometimes catastrophically.
Why Combine Medications in the First Place?
Many people don’t get better on just one antidepressant. The STAR*D trial, a massive study by the National Institute of Mental Health, found that nearly 40% of people with major depression didn’t respond to their first medication. That’s not a failure of the patient-it’s a failure of the one-size-fits-all approach. So doctors turn to combinations. The most common? An SSRI or SNRI like escitalopram or sertraline paired with a low-dose atypical antipsychotic like aripiprazole (Abilify). This combo isn’t random. Aripiprazole works on dopamine and serotonin in a way that helps lift mood without causing the sedation or weight gain of older antipsychotics. The FDA approved this specific combination in 2014 after trials showed it boosted remission rates from 11% to nearly 25%. Other common pairings include:- Symbyax (olanzapine + fluoxetine): A single pill with two active ingredients, approved in 2003 for treatment-resistant depression.
- SSRI + bupropion: Used to fix sexual side effects from SSRIs-studies show 60-70% of patients see improvement.
- SSRI + buspirone: For lingering anxiety without the risk of addiction that comes with benzodiazepines.
The Generic Switch: A Silent Risk
The FDA says generics are just as good as brand names. They must contain the same active ingredient and be 80-125% as bioavailable. Sounds fair, right? Not when you’re dealing with psychiatric meds. Psychiatric drugs often have a narrow therapeutic window. That means the difference between a dose that works and one that causes harm is tiny. Lithium, for example, must stay between 0.6 and 1.2 mmol/L. Go below that, and depression returns. Go above, and you risk tremors, confusion, or even kidney damage. A 2018 case series from the University of British Columbia tracked three bipolar patients who switched from brand-name Eskalith (lithium carbonate) to a generic. Their lithium levels dropped from 0.85 to 0.55 mmol/L-even though their dose didn’t change. Within two weeks, all three relapsed into mania. It’s not just lithium. A 2019 study of over 28,000 patients found that switching from brand-name SSRIs to generics led to a 22.3% higher chance of treatment failure. That’s not a small number. That’s one in five people who suddenly feel worse after a pharmacy substitution they didn’t even know was happening.Why Some Generics Are Riskier Than Others
Not all generics are created equal. The problem isn’t just the active ingredient-it’s how the drug is released into your body. Take venlafaxine ER (Effexor XR). It’s designed to release serotonin and norepinephrine in a 2:1 ratio over time. But different generic manufacturers use different bead technologies. One might release the drug too fast. Another too slow. That changes the balance. If you’re on venlafaxine plus an SSRI, that shift can destabilize your entire treatment. Then there’s bupropion XL (Wellbutrin XL). In 2012, the FDA issued a warning after 137 reports of patients experiencing sudden anxiety, panic attacks, or depression after switching to a generic version. The issue? Inconsistent drug release. Some batches released too much too fast. Others didn’t release enough. The result? Mood swings, insomnia, and in some cases, hospitalizations. Even Lamictal (lamotrigine), used for bipolar disorder, has had problems. Patients on Reddit and PatientsLikeMe report that switching from brand to generic-especially Apotex or Mylan versions-caused their mood to crash. One user wrote: “My Zoloft stopped working after the switch. I didn’t change anything else. Just the generic Lamictal.”
What the Experts Are Saying
The American Psychiatric Association’s 2022 guidelines don’t mince words: “Switching between generic manufacturers may be as problematic as switching from brand to generic.” Dr. Joseph Goldberg from Mount Sinai found that patients on combination therapy with lithium had a 34% higher risk of hospitalization after a generic switch. Dr. Charles Popkin at New York-Presbyterian called the FDA’s 80-125% bioequivalence range “unacceptable” for psychiatric drugs used in combination. Why? Because these drugs aren’t just floating around in your bloodstream-they’re fine-tuning brain chemistry. A 10% change in absorption might not matter for an antibiotic. But for a drug that controls your mood, it can mean the difference between stability and crisis. Even those who support generics for cost reasons admit the risk. Dr. G. Caleb Alexander from Johns Hopkins says: “Specific high-risk combinations, particularly those involving lithium or clozapine, warrant closer monitoring following substitution.”Real Patients, Real Consequences
Online forums are full of stories that clinical trials never capture. On r/depression, a thread titled “Generic switch ruined my carefully balanced med cocktail” got over 1,200 upvotes. People wrote about:- Obsessive thoughts returning after switching Abilify generics.
- Severe akathisia (inner restlessness) after switching fluoxetine.
- Worsening panic attacks after changing venlafaxine brands.
How to Protect Yourself
If you’re on a psychiatric combination, here’s what you can do:- Ask your doctor to write “Dispense as Written” or “Do Not Substitute” on your prescription. This legally prevents the pharmacist from swapping your brand for a generic without approval.
- Know your manufacturer. If you’re on a generic, write down the name on the pill bottle-Aurobindo, Teva, Mylan, etc. If you see a different name next refill, ask why.
- Track your symptoms. Use a simple journal: rate your mood, sleep, anxiety, and side effects on a scale of 1-10 every day for two weeks after any switch.
- Request therapeutic drug monitoring. For lithium, valproate, or clozapine, ask for a blood test 7-14 days after any generic change. Even a small drop in levels can trigger relapse.
- Don’t wait. If you feel worse within 10 days of a switch, call your prescriber immediately. Don’t assume it’s “just adjustment.”
The Bigger Picture: Cost vs. Safety
Generics saved the U.S. healthcare system over $300 billion in 2022. That’s huge. But when it comes to psychiatric combinations, the cost of a relapse is higher than the savings. A 2023 Congressional Budget Office report estimates that without changes, avoidable hospitalizations from bad generic switches will cost $2.4 billion annually by 2027. Some states are acting. California’s AB 1477, effective January 2023, requires pharmacists to notify doctors when switching psychotropic generics in patients on multiple medications. Michigan saw a 22% drop in ER visits after passing a similar law. And now, authorized generics-brand-name drugs sold under a generic label-are becoming more common. Symbyax’s authorized generic (olanzapine/fluoxetine) launched in 2022. It’s the same drug, same manufacturer, just cheaper. That’s the ideal solution.What’s Next?
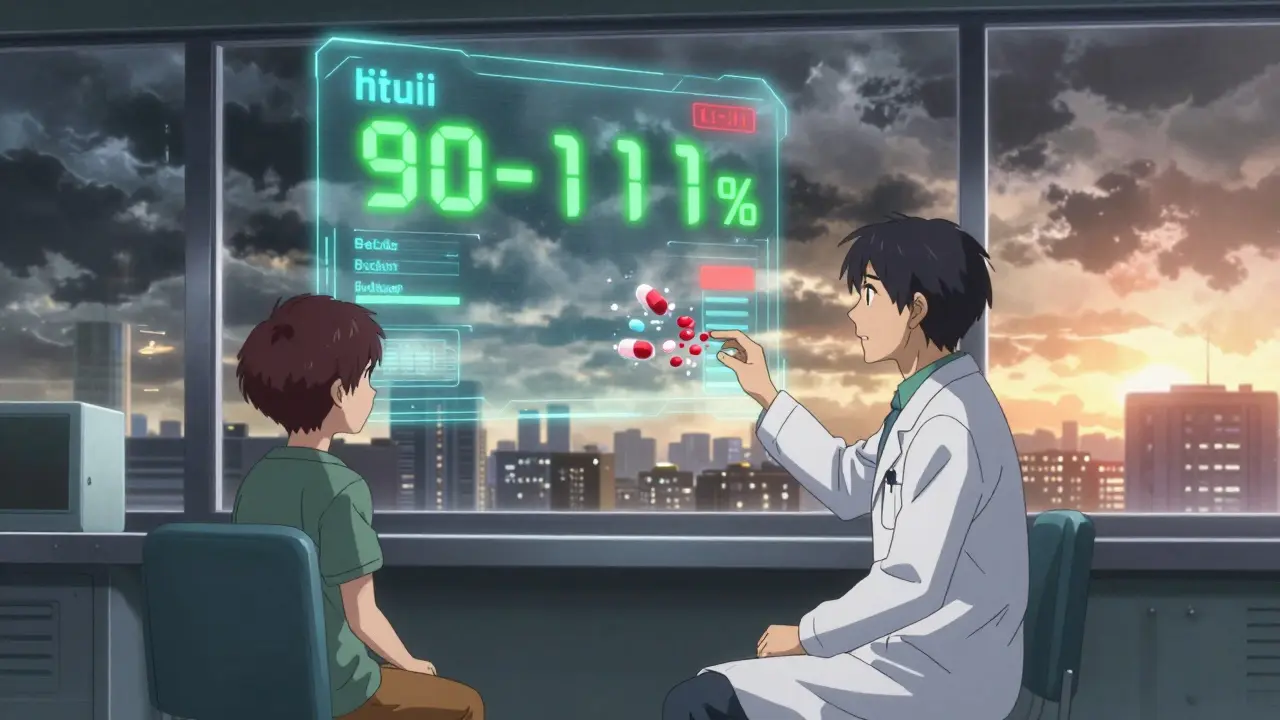
The FDA is finally listening. In May 2023, they proposed narrowing the bioequivalence range for extended-release psychiatric drugs to 90-111%. That’s a big step. By 2025, they plan to implement product-specific guidelines for 27 high-risk combinations. Long-term, experts believe pharmacogenetic testing-analyzing how your genes affect drug metabolism-will guide which generic you get. That could cut adverse outcomes by 60%. But for now, the responsibility falls on you and your doctor. Don’t assume a generic is safe just because it’s cheaper. Don’t assume your pharmacist knows the difference between bead technologies or release profiles. And don’t wait until you’re in crisis to speak up. Your mental health isn’t a commodity. It’s not a line item on a balance sheet. It’s your ability to wake up, connect, work, and live. And that’s worth protecting.Can I switch from brand-name psychiatric meds to generics safely?
It depends. For single medications like sertraline or fluoxetine, many people switch without issue. But for combination therapy-especially with lithium, lamotrigine, bupropion XL, or venlafaxine ER-the risk of relapse or side effects increases significantly. Always consult your doctor before switching, and never allow automatic substitution without your explicit consent.
Why do generics sometimes make psychiatric symptoms worse?
Generics must meet FDA bioequivalence standards (80-125% of the brand’s absorption), but that’s a wide range. For psychiatric drugs with narrow therapeutic windows, even a 15% drop in absorption can cause symptoms to return. Differences in inactive ingredients, bead technology, or release rates can alter how the drug works in your brain, especially when combined with other medications.
Which psychiatric generics are most likely to cause problems?
Generic versions of bupropion XL, venlafaxine ER, lithium carbonate, lamotrigine, and carbamazepine have the highest reports of issues. These drugs have complex release systems or require precise blood levels. Generic substitutions for these have triggered relapses, mania, akathisia, and hospitalizations in documented cases.
Should I ask my doctor to write “Dispense as Written”?
Yes-if you’re on a combination therapy, especially with a narrow therapeutic index drug. This legally prevents the pharmacy from substituting a generic without your prescriber’s approval. Many patients don’t know they have this right. Use it.
Are authorized generics safer than regular generics?
Yes. Authorized generics are made by the original brand-name manufacturer and sold under a generic label. They’re chemically identical to the brand, with no variation in formulation or release. If your medication has an authorized generic option (like Symbyax’s version), ask your doctor to prescribe it-it’s the safest generic choice.
How long should I wait before deciding if a generic is working?
Don’t wait more than 10 days. If you notice increased anxiety, mood swings, insomnia, or return of depressive symptoms within that window, contact your prescriber immediately. Unlike some medications that take weeks to adjust, psychiatric combos can destabilize quickly after a substitution.
Can I request therapeutic drug monitoring for my psychiatric meds?
Yes, and you should-especially if you’re on lithium, valproate, carbamazepine, clozapine, or any combination involving these. Blood tests 7-14 days after a generic switch can catch dangerous drops or spikes in levels before symptoms worsen. This isn’t routine, but it’s critical for safety.


Frank SSS
January 1, 2026 AT 11:59Man, I switched my Abilify generic last month and thought I was just having a bad week. Turned out my lithium levels dropped like a rock. Had to go back to brand. Don't let the pharmacy play Russian roulette with your brain.
Also, why do they even make generics for stuff like this? It's not like we're treating acne.
Hanna Spittel
January 2, 2026 AT 15:38THEY KNOW. They *know* generics mess with your head. Big Pharma doesn't want you stable-they want you hooked on $$$.
👀💊 #PharmaCoverUp
Brady K.
January 4, 2026 AT 06:39Let’s be real-the FDA’s 80-125% bioequivalence range for psychiatric meds is a joke. It’s like saying two different engines are interchangeable if they both produce between 200 and 312 horsepower. One might get you to work. The other might launch you into orbit-or into the ER.
They treat brain chemistry like it’s a commodity, not a symphony. And when you throw in multiple drugs with complex release profiles? You’re not just risking relapse-you’re gambling with someone’s ability to function, to love, to breathe without panic.
And yet, we still let pharmacists swap these without consent? That’s not healthcare. That’s corporate negligence dressed up as cost-saving.
It’s not ‘generic’ because it’s cheaper. It’s generic because they stopped caring about the human being on the other end of the prescription.
Meanwhile, authorized generics exist. Why aren’t we pushing those as the standard? Because the system doesn’t reward safety. It rewards margins.
And if you’re not screaming about this? You’re complicit.
Retha Dungga
January 5, 2026 AT 12:11we are all just chemicals in a jar really 🤔
but when the jar gets swapped for a cheaper one... who are we then 🫠
the system is broken but so are we right? 🤷♀️
maybe the real med is connection not pills 🌱
Jenny Salmingo
January 5, 2026 AT 15:13I’ve been on a combo for years and never knew I could ask for ‘Dispense as Written.’ I’m calling my doctor tomorrow. Thank you for this post. 💙
Aaron Bales
January 5, 2026 AT 17:59If you’re on lithium, lamotrigine, or bupropion XL-don’t let them switch your generic without a blood test. Period.
And if your pharmacy says ‘it’s the same thing,’ ask them to show you the FDA’s product-specific guidelines for that exact formulation. They can’t. Because there aren’t any yet.
Lawver Stanton
January 6, 2026 AT 16:21Okay but let’s talk about how insane it is that we’re still having this conversation in 2025. I’ve been on the same combo since 2018-brand name, no changes. My therapist says I’m one of the few stable ones in the whole clinic. Why? Because I refused every single generic switch. Every. Single. One.
I’ve had pharmacists try to argue with me. One even said, ‘It’s just a pill.’ Just a pill? Bro, I’ve been in the hospital twice because of a generic switch. Twice. One time I was so akathisic I tried to climb out the window. I didn’t want to die-I just couldn’t sit still anymore.
And now I’m supposed to trust a $0.10 difference in cost? No. No. No.
I’ve written letters to my state reps. I’ve called the FDA. I’ve posted on every subreddit I can find. I’ve even had my doctor call the pharmacy directly and scream into the phone. It’s exhausting. But I’d rather be exhausted than dead.
And if you think this doesn’t affect you? You’re either on a single med and lucky-or you’re lying to yourself. The system is rigged. The FDA’s standards are outdated. The manufacturers don’t care. And your pharmacist? They’re just following the script.
So what do you do? You fight. You demand. You refuse. You ask for the authorized generic. You demand blood tests. You track your symptoms. You don’t wait 10 days-you call at day 3 if something feels off.
Because your brain isn’t a commodity. It’s your whole damn life.
Paul Huppert
January 8, 2026 AT 08:06Has anyone here tried the authorized generic for Symbyax? I got mine last month and my mood’s been rock solid. No weird anxiety spikes, no insomnia. Just… steady. Weird how the exact same pills, just under a different label, made all the difference.
Kayla Kliphardt
January 9, 2026 AT 13:49My mom’s on lamotrigine and lithium. She switched generics last year and didn’t tell anyone until she cried during dinner one night. We found out because her handwriting changed-tiny, shaky letters. That’s how I knew something was wrong.
Now we always check the pill bottle. Always. And she gets bloodwork every 6 weeks. It’s a hassle. But worth it.