For many people with chronic spontaneous urticaria (CSU), life doesn’t stop when the hives come and go. It’s not just about itchy skin-it’s sleepless nights, canceled plans, anxiety in public, and the frustration of seeing doctor after doctor without real relief. Even when first-line treatments like second-generation antihistamines are used at full dose, 60% of patients still don’t get enough control. That’s where second-line treatments come in. These aren’t backup options-they’re often the turning point between ongoing suffering and real quality of life.
Why First-Line Treatments Often Fail
Most people start with antihistamines like cetirizine, loratadine, or fexofenadine. These work well for acute hives, but CSU is different. It’s not caused by allergens you can avoid. It’s driven by your own immune system-sometimes by autoantibodies that attack your mast cells, making them release histamine for no reason. About 40% of patients get decent relief with standard doses. That leaves the other 60% stuck. Some doctors will bump the dose up to two, three, or even four times the normal amount. But even then, studies show only about 40% of patients overall get meaningful improvement. That’s not enough. When your skin is breaking out daily, you need something stronger.Omalizumab: The Current Gold Standard
Omalizumab is the most widely used second-line treatment for CSU. It’s not a steroid. It’s not an immunosuppressant. It’s a monoclonal antibody that binds to IgE, the antibody that triggers mast cells to release histamine. By mopping up free IgE, it stops the chain reaction before it starts. Patients get a subcutaneous injection every four weeks-no daily pills, no complex dosing. In clinical trials, about 30-70% of patients saw a major drop in hives and itching. But here’s the catch: 70% of patients still don’t achieve complete control. And for those with IgG-mediated autoimmune CSU-about 30-50% of all cases-omalizumab often doesn’t work at all. That’s not a failure of the drug. It’s a failure of the one-size-fits-all approach.The New Contenders: Remibrutinib and Dupilumab
The treatment landscape is changing fast. Two new drugs are emerging as serious alternatives-and they’re both oral, which changes everything. Remibrutinib is a Bruton tyrosine kinase inhibitor (BTKi). It doesn’t just block IgE. It hits multiple pathways: it calms down mast cells, reduces basophil activity, and lowers the production of autoantibodies that attack your skin. In two large phase 3 trials (REMIX-1 and REMIX-2), 28-32% of patients achieved complete symptom control after 24 weeks. That’s comparable to omalizumab, but with a pill instead of an injection. For someone juggling work, kids, or travel, that convenience matters. Adherence skyrockets when you don’t need to schedule monthly clinic visits. Dupilumab works differently. It blocks IL-4 and IL-13, two key inflammatory signals involved in allergic and autoimmune skin reactions. In trials, 30-31% of CSU patients had complete clearance of hives and itching by week 24. That’s slightly better than omalizumab’s average. The problem? It’s not yet approved for CSU. It’s approved for eczema and asthma, and doctors are using it off-label. But with strong data behind it, approval is expected soon. For patients who’ve tried everything else, this could be a game-changer.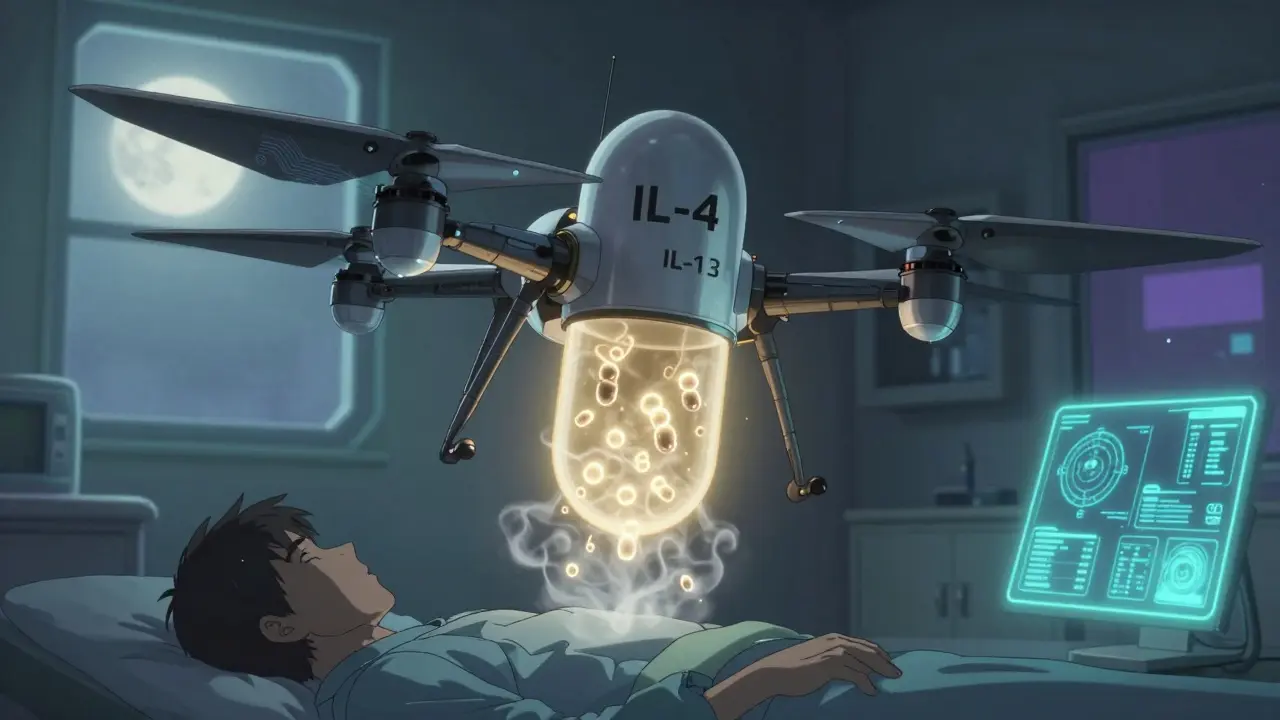
What About Cyclosporine?
Cyclosporine is an older drug, originally used for organ transplant patients. It’s powerful. In autoimmune CSU cases, it works for 54-73% of people. That’s higher than omalizumab in this subgroup. But it comes with serious risks: high blood pressure, kidney damage, and the need for frequent blood tests. Most doctors use it only after biologics fail, or when biologics aren’t available. It’s not a long-term solution, but for someone with severe, uncontrolled hives, it can be a bridge to something better.Why Some Drugs Failed: The Fenebrutinib Lesson
Not every new drug makes it. Fenebrutinib, another BTK inhibitor, showed promise in early trials. But during phase 3 testing, a portion of patients developed elevated liver enzymes-signs of liver stress. The program was halted in 2023. This isn’t a failure of science. It’s a reminder that safety is non-negotiable. Even if a drug works better, if it risks your liver or other organs, it won’t be approved. That’s why remibrutinib and dupilumab are being watched so closely: their safety profiles look clean so far.
Choosing the Right Treatment for You
There’s no universal answer. Your best option depends on your type of CSU. If you have IgG autoantibodies (which can be tested), omalizumab is likely to disappoint. Cyclosporine or a BTK inhibitor like remibrutinib might be better. If your skin flares are tied to inflammation beyond histamine-like redness, swelling, or persistent discomfort-dupilumab could be the right fit. And if you just need something reliable and proven, omalizumab still holds the top spot. Here’s what to ask your doctor:- Have I been tested for autoimmune markers in CSU?
- Have I tried antihistamines at the maximum dose for at least 4 weeks?
- Am I a candidate for an oral treatment, or do I need injections?
- What are the risks of long-term use for each option?
- Is there a way to track my progress beyond just counting hives-like using a daily symptom diary?
What’s Next for CSU Treatment?
The future is personalization. We’re moving away from treating all CSU the same. Researchers now believe CSU isn’t one disease-it’s several. Some are IgE-driven. Others are IgG-driven. Some involve T-cells. Others involve mast cell overactivity. Within the next 3-5 years, we’ll likely see tests that tell you which subtype you have-and match you to the drug that works best for your biology. That’s the real goal: not just controlling symptoms, but stopping the disease at its source. For now, the options are better than ever. If you’ve been told, ‘You’ll just have to live with it,’ that’s no longer true. Second-line treatments exist. They work. And more are coming. The key is knowing what’s out there-and asking for the right test, the right drug, the right path forward.What is the difference between first-line and second-line treatments for chronic spontaneous urticaria?
First-line treatments are second-generation antihistamines like cetirizine or fexofenadine, taken daily to block histamine. They work for about 40% of people with chronic spontaneous urticaria (CSU). Second-line treatments are used when those don’t work enough. These include biologics like omalizumab (an injection), oral drugs like remibrutinib, or off-label options like cyclosporine. Second-line options target the immune system more directly and are meant for patients with ongoing, severe symptoms.
Why doesn’t omalizumab work for everyone with CSU?
Omalizumab blocks IgE, which triggers hives in some patients. But about 30-50% of CSU cases are driven by IgG autoantibodies, not IgE. These autoantibodies activate mast cells directly, bypassing IgE entirely. Since omalizumab doesn’t affect IgG, it has little to no effect on this subgroup. That’s why some patients see great results and others see nothing-even though they have the same symptoms.
Is remibrutinib better than omalizumab?
Remibrutinib and omalizumab have similar effectiveness-both help about 30% of patients achieve complete symptom control. But remibrutinib is taken as a daily pill, while omalizumab requires monthly injections. For many, convenience means better adherence. Remibrutinib also targets multiple immune pathways, including IgG-driven inflammation, which may make it more effective for patients who don’t respond to omalizumab. It’s not approved yet, but it’s expected to be soon.
Can I take dupilumab for chronic spontaneous urticaria right now?
Dupilumab is not yet approved specifically for CSU, but it’s being used off-label by many specialists. It’s FDA-approved for eczema and asthma, and phase 3 trials for CSU showed 30-31% of patients had complete clearance of hives and itching. If your doctor believes you’re a good candidate-especially if you have persistent inflammation beyond itching-they may prescribe it. Insurance coverage can be tricky, but many patients get approval with proper documentation.
How do I know if I have autoimmune chronic spontaneous urticaria?
There’s a simple blood test called the autologous serum skin test (ASST) that can suggest autoimmune CSU. It checks if your own serum triggers a hive reaction when injected under your skin. Another test looks for IgG autoantibodies against the IgE receptor. Not all clinics offer these, but if you’ve tried multiple treatments without success, ask your allergist or dermatologist about testing. Knowing your subtype helps pick the right second-line treatment.
What are the side effects of remibrutinib and dupilumab?
Remibrutinib’s most common side effects are mild: headache, upper respiratory infection, and nausea. Serious side effects are rare in trials. Dupilumab is well-tolerated too-most people get mild injection-site reactions, conjunctivitis (eye inflammation), or cold sores. Neither causes liver damage, kidney issues, or immune suppression like cyclosporine. Both are considered safer than older immunosuppressants.
How long does it take for second-line treatments to work?
Omalizumab usually shows improvement within 4-8 weeks, with full effect by 12-16 weeks. Remibrutinib and dupilumab show noticeable results around week 4, with peak results at week 12-24. Unlike antihistamines, which work within hours, these drugs fix the root cause, so they take longer-but the results last longer too. Don’t stop too early. Give it at least 3 months before deciding if it’s working.
Can I switch from omalizumab to remibrutinib if it doesn’t work?
Yes. Many patients who don’t respond to omalizumab go on to remibrutinib or dupilumab. In fact, that’s exactly how these new drugs are being studied-in patients who failed biologics. There’s no waiting period between stopping one and starting another. Your doctor will monitor you closely, but switching is common and safe. The goal is to find what works for your body, not to stick with the first option.




Hannah Taylor
December 21, 2025 AT 01:52Sandy Crux
December 21, 2025 AT 16:42Jon Paramore
December 22, 2025 AT 06:48Christina Weber
December 22, 2025 AT 11:17Sarah Williams
December 23, 2025 AT 11:34John Hay
December 25, 2025 AT 09:13mukesh matav
December 27, 2025 AT 03:41Jay lawch
December 27, 2025 AT 23:45Peggy Adams
December 29, 2025 AT 17:39Swapneel Mehta
December 30, 2025 AT 07:40Theo Newbold
December 31, 2025 AT 17:48